Osmotic Pressure Calculator
Osmotic pressure calculator to find the osmotic pressure of a solution containing dissolved solids. The Osmotic pressure arises when a pure solvent and a solution or two solutions of different concentrations.
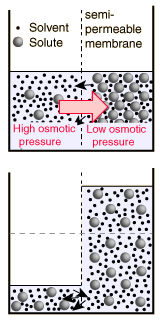
Osmotic pressure formula
π = i . Φ * c . R. T
Where πis Osmotic pressure, T is the temperature on the Kelvin scale, R is 0.083145 Liter.bar/(deg.mol) the Universal gas constant, i is Number of ions produced by solute dissiation, e.g. 3 of MgCl2 2 of NaCl, c is Concentration of the solution, Φ is Osmotic coeffient.
For example, when Number of ions is 3, Osmotic Coefficient is 0.2, Concentration of all solutes is 3, Absolute Temperature is 20, then Osmotic Pressure is 2.99322 Bar.
Thinkcalculator.com provides you helpful and handy calculator resources.
Word Counter | AllCallers | CallerInfo | ThinkCalculator | Free Code Format
Copyright ©2006 - 2024 Thinkcalculator All Rights Reserved. Contact Us: [email protected]